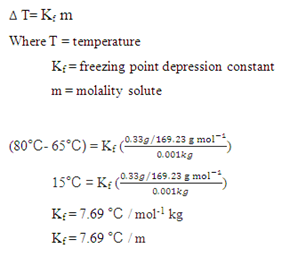
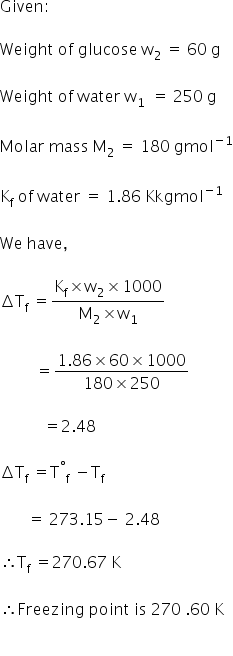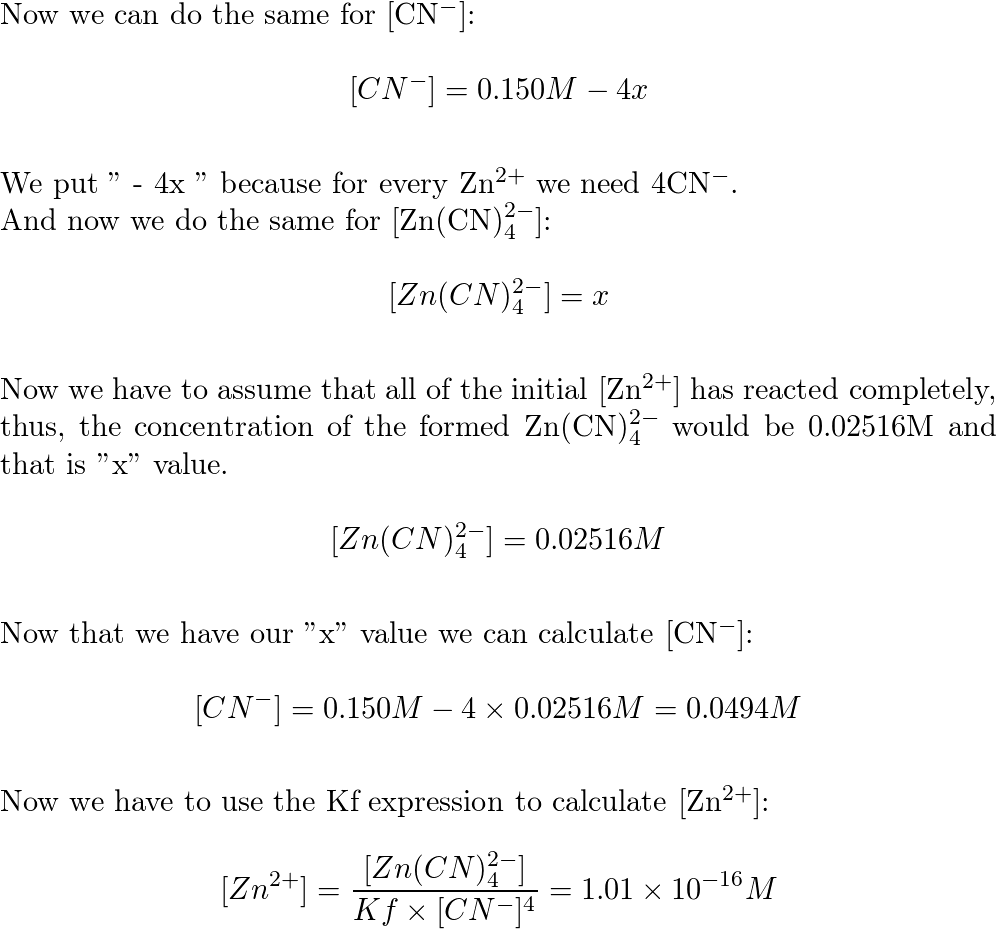After you preform your experiment, you determine that the Kf value for naphthalene is 6.9 . You are using 10g of naphthalene and added 1.0 g of your unknown. The the freezing

Calculate the amount of KCl which must be added to 1 kg of water so that the freezing point is depressed by 2K. (kf for water = 1.86 K kg mol^-1)

Calculate the freezing point of a solution containing 60 g of glucose (Molar mass = 180 g mol^-1 ) in 250 g of water. ( Kf of water = 1.86 K kg mol^-1 ).
Using the following energy values determine the lattice energy of KF(s) - Sarthaks eConnect | Largest Online Education Community
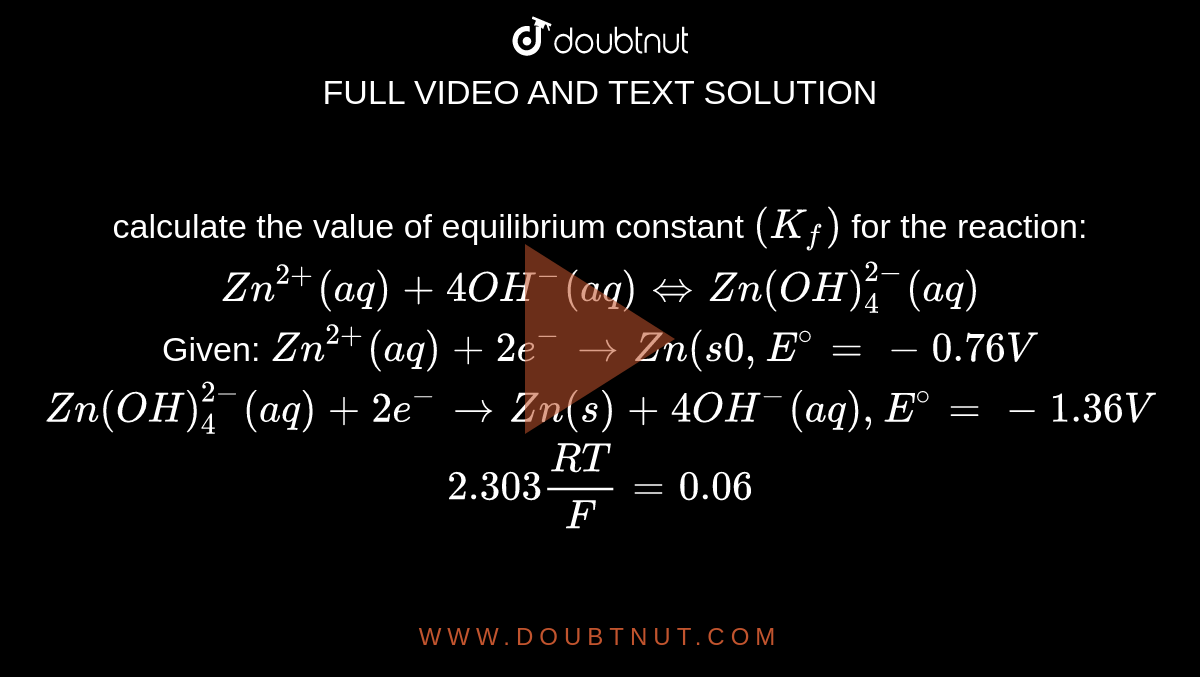
calculate the value of equilibrium constant (Kf) for the reaction: Zn^(2+)(aq)+4OH^(-)(aq)iffZn(OH)4^(2-)(aq) Given: Zn^(2+)(aq)+2e^(-)toZn(s0,E^(@)=-0.76 V Zn(OH)4^(2-)(aq)+2e^(-)toZn(s)+4OH^(-)(aq),E^(@)=-1.36 V 2.303(RT)/F=0.06

Calculate the lattice energy of potassium fluoride, KF, using the Born-Haber cycle. Use thermodynamic data to obtain the enthalpy changes for each step. | Homework.Study.com

Calculate Complex Ion Equilibria Using the Small x Approximation for Large Kf | Chemistry | Study.com

After you preform your experiment, you determine that the Kf value for naphthalene is 6.9 . You are using 10g of naphthalene and added 1.0 g of your unknown. The the freezing

How are Kf values relevant in calculations of the melting temperature of a solution? | Homework.Study.com
![Calculate the depression in the freezing point of water when 10 g of CH3CH2CHClCOOH is added to 250 g of water. [ Ka = 1.4 × 10^-3, Kf = 1.86 K kg mol^-1 ] Calculate the depression in the freezing point of water when 10 g of CH3CH2CHClCOOH is added to 250 g of water. [ Ka = 1.4 × 10^-3, Kf = 1.86 K kg mol^-1 ]](https://i.ytimg.com/vi/zGfIbhioFZ0/mqdefault.jpg)